These highlights do not include all the information needed to use AFREZZA ® safely and effectively. See full prescribing information for AFREZZA.
AFREZZA ® (insulin human) inhalation powder, for oral inhalation use
Initial U.S. Approval: 2014
AFREZZA ® is a rapid acting inhaled human insulin indicated to improve glycemic control in adult patients with diabetes mellitus. (1)
Limitations of Use:
Inhalation powder in single-use cartridges of: 4 units, 8 units, or 12 units (3)
The most common adverse reactions associated with AFREZZA (2% or greater incidence) are hypoglycemia, cough, and throat pain or irritation (6)
To report SUSPECTED ADVERSE REACTIONS, contact MannKind at 1-877-323-8505 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
AFREZZA is contraindicated in patients with chronic lung disease because of the risk of acute bronchospasm in these patients. Before initiating AFREZZA, perform a medical history, physical examination and spirometry (FEV 1 ) in all patients to identify potential lung disease [see Contraindications (4) and Warnings and Precautions (5.1)].
Only administer AFREZZA via oral inhalation using the AFREZZA Inhaler. Administer AFREZZA at the beginning of each meal. Administer AFREZZA using a single inhalation per cartridge (if the dose is greater than the contents of a single cartridge, more than one cartridge is needed) [see Dosage and Administration (2.3)]. For additional administration instructions on how to use the AFREZZA Inhaler [see Dosage and Administration (2.6)] and see the Instructions for Use. The AFREZZA Inhaler can be used for up to 15 days from the date of first use. After 15 days of use, the inhaler must be discarded and replaced with a new inhaler.
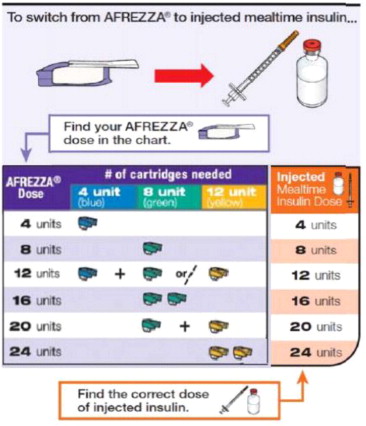
For patients using subcutaneous, pre-mixed insulin :
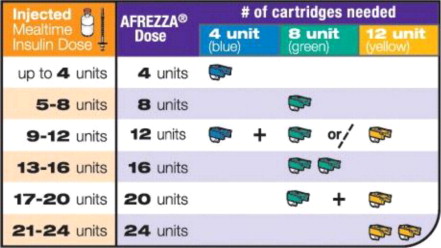
For AFREZZA doses exceeding the contents of a single cartridge at mealtime, inhalations from more than one cartridge are necessary. To achieve the required total mealtime dose, use a combination of 4 unit, 8 unit, and 12 unit cartridges. Examples of cartridge combinations for doses of up to 24 units are shown in Figure 1. For doses above 24 units, use combinations of different multiple cartridges.
Figure 1. Mealtime AFREZZA Starting Dose Conversion Table

Dosage modification may be needed when:
Refer patients to the Instructions for Use for detailed instructions and visuals on how to prepare, administer, and store AFREZZA; use the AFREZZA cartridges; and use the AFREZZA inhaler. Critical administration instructions are as follows:
Inhalation Powder: single-use cartridges containing 4 units, 8 units or 12 units of insulin human as white powder to be administered via oral inhalation with the AFREZZA inhaler only [see How Supplied/Storage and Handling (16)] .
Because of the risk of acute bronchospasm, AFREZZA is contraindicated in patients with chronic lung disease such as asthma or COPD [see Contraindications (4)] . Before initiating therapy with AFREZZA, evaluate all patients with a medical history, physical examination, and spirometry (FEV 1 ) to identify potential underlying lung disease. Acute bronchospasm has been observed in AFREZZA-treated patients with asthma and COPD. In a study of patients with asthma whose bronchodilators were temporarily withheld for assessment, bronchoconstriction and wheezing following AFREZZA dosing was reported in 29% (5 out of 17) and 0% (0 out of 13) of patients with and without a diagnosis of asthma, respectively. In this study, a mean decline in FEV 1 of 400 mL was observed 15 minutes after a single AFREZZA dose in patients with asthma. In a subset study of patients with COPD (n=8), a mean decline in FEV 1 of 200 mL was observed 18 minutes after a single AFREZZA dose.
Glucose monitoring is essential for patients receiving insulin therapy. Changes in insulin strength, manufacturer, type, or method of administration may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions (5.3)] or hyperglycemia. These changes should be made under close medical supervision and the frequency of blood glucose monitoring should be increased. For patients with type 2 diabetes, dosage modifications of concomitant oral antidiabetic treatment may be needed [see Drug Interactions (7.1, 7.2, and 7.3)] .
Glucose monitoring is essential for patients receiving insulin therapy. Hypoglycemia is the most common adverse reaction associated with insulins, including AFREZZA. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). The timing of hypoglycemia usually reflects the time-action profile of the administered insulin formulation. AFREZZA has a distinct time action profile [see Clinical Pharmacology (12)] , which impacts the timing of hypoglycemia. Hypoglycemia can happen suddenly, and symptoms may differ across patients and change over time in the same patient. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic nerve disease, in patients using certain medications [see Drug Interactions (7.4)] , or in patients who experience recurrent hypoglycemia. Factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content or timing of meals), changes in level of physical activity, or changes to co-administered medication [see Drug Interactions (7)] . Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [see Use in Specific Populations (8.6, 8.7)] .
Risk Mitigation Strategies for Hypoglycemia Patients and caregivers should be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.
AFREZZA causes a decline in pulmonary function over time as measured by FEV 1 . In clinical trials excluding patients with chronic lung disease and lasting up to 2 years, AFREZZA-treated patients experienced a small [40 mL (95% CI: -80, -1)] but greater FEV 1 decline than comparator-treated patients. The FEV 1 decline was noted within the first 3 months, and persisted for the entire duration of therapy (up to 2 years of observation). In this population, the annual rate of FEV 1 decline did not appear to worsen with increased duration of use. The effects of AFREZZA on pulmonary function for treatment duration longer than 2 years has not been established. There are insufficient data in long term studies to draw conclusions regarding reversal of the effect on FEV 1 after discontinuation of AFREZZA. The observed changes in FEV 1 were similar in patients with type 1 and type 2 diabetes. Assess pulmonary function (e.g., spirometry) at baseline, after the first 6 months of therapy, and annually thereafter, even in the absence of pulmonary symptoms. In patients who have a decline of ≥ 20% in FEV 1 from baseline, consider discontinuing AFREZZA. Consider more frequent monitoring of pulmonary function in patients with pulmonary symptoms such as wheezing, bronchospasm, breathing difficulties, or persistent or recurring cough. If symptoms persist, discontinue AFREZZA [see Adverse Reactions (6)] .
In clinical trials, two cases of lung cancer, one in controlled trials and one in uncontrolled trials (2 cases in 2,750 patient-years of exposure), were observed in patients exposed to AFREZZA while no cases of lung cancer were observed in patients exposed to comparators (0 cases in 2,169 patient-years of exposure). In both cases, a prior history of heavy tobacco use was identified as a risk factor for lung cancer. Two additional cases of lung cancer (squamous cell and lung blastoma) occurred in non-smokers exposed to AFREZZA and were reported by investigators after clinical trial completion. These data are insufficient to determine whether AFREZZA has an effect on lung or respiratory tract tumors. In patients with active lung cancer, a prior history of lung cancer, or in patients at risk for lung cancer, consider whether the benefits of AFREZZA use outweigh this potential risk.
In clinical trials enrolling patients with type 1 diabetes, diabetic ketoacidosis (DKA) was more common in AFREZZA-treated patients (0.43%; n=13) than in comparator-treated patients (0.14%; n=3). Patients with type 1 diabetes should always use AFREZZA in combination with basal insulin. In patients at risk for DKA, such as those with an acute illness or infection, increase the frequency of glucose monitoring and consider discontinuing AFREZZA and giving insulin using an alternate route of administration.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including AFREZZA. If hypersensitivity reactions occur, discontinue AFREZZA, treat per standard of care and monitor until symptoms and signs resolve [see Adverse Reactions (6)] . AFREZZA is contraindicated in patients who have had hypersensitivity reactions to AFREZZA or any of its excipients [see Contraindications (4)] .
All insulin products, including AFREZZA, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in AFREZZA-treated patients at risk for hypokalemia (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentrations and patients receiving intravenously administered insulin).
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including AFREZZA, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist should be considered.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure of 3,017 patients to AFREZZA and include 1,026 patients with type 1 diabetes and 1,991 patients with type 2 diabetes. The mean exposure duration was 8.2 months for patients with type 1 diabetes and those with type 2 diabetes. In the overall population:
AFREZZA was studied in placebo and active-controlled trials (n = 3 and n = 10, respectively).
The mean age of the population was 50.2 years and 20 patients were older than 75 years of age; 51% of the population were males; 83% were White, 5% were Black or African American, and 2% were Asian; 10% were Hispanic. At baseline, the type 1 diabetes population had diabetes for an average of 16.6 years and had a mean HbA1c of 8.3%, and the type 2 diabetes population had diabetes for an average of 10.7 years and had a mean HbA1c of 8.8%. At baseline, 33% of the population reported peripheral neuropathy, 32% reported retinopathy and 20% had a history of cardiovascular disease.
Table 1 shows the frequency of common adverse reactions, excluding hypoglycemia, associated with the use of AFREZZA in the pool of controlled trials in type 2 diabetes patients. These adverse reactions were not present at baseline, occurred more commonly on AFREZZA than on placebo and/or comparator and occurred in at least 2% of patients treated with AFREZZA.
*Carrier particle without insulin was used as placebo [see Description (11.1)] .
Table 2 shows the frequency of common adverse reactions, excluding hypoglycemia, associated with the use of AFREZZA in the pool of active-controlled trials in type 1 diabetes patients. These adverse reactions were not present at baseline, occurred more commonly on AFREZZA than on comparator, and occurred in at least 2% of patients treated with AFREZZA.
| AFREZZA (n=1026) | Subcutaneous Insulin (n = 835) | |
| Cough | 29.4% | 4.9% |
| Throat pain or irritation | 5.5% | 1.9% |
| Headache | 4.7% | 2.8% |
| Pulmonary function test decreased | 2.8% | 1.0% |
| Bronchitis | 2.5% | 2.0% |
| Urinary tract infection | 2.3% | 1.9% |
Hypoglycemia is the most commonly observed adverse reaction in patients using insulin, including AFREZZA [see Warnings and Precautions (5.3)] . The incidence of severe and non-severe hypoglycemia in AFREZZA-treated patients versus placebo-treated patients with type 2 diabetes is shown in Table 3. A hypoglycemic episode was recorded if a patient reported symptoms of hypoglycemia with or without a blood glucose value consistent with hypoglycemia. Severe hypoglycemia was defined as an event with symptoms consistent with hypoglycemia requiring the assistance of another person and associated with either a blood glucose value consistent with hypoglycemia or prompt recovery after treatment for hypoglycemia.
| AFREZZA (N=177) | Placebo (N=176) | |
| Severe Hypoglycemia | 5.1% | 1.7% |
| Non-Severe Hypoglycemia | 67% | 30% |
Approximately 27% of patients treated with AFREZZA reported cough, compared to approximately 5% of patients treated with comparator. In clinical trials, cough was the most common reason for discontinuation of AFREZZA therapy (3% of AFREZZA-treated patients).
Pulmonary Function Decline
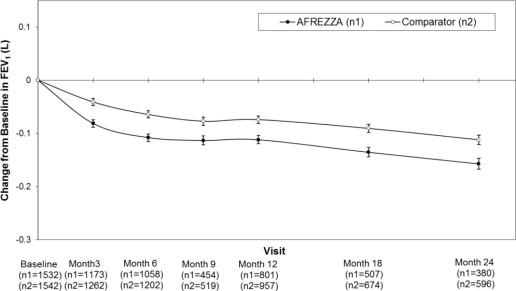
In clinical trials lasting up to 2 years, excluding patients with chronic lung disease, patients treated with AFREZZA had a 40 mL (95% CI: -80, -1) greater decline from baseline in forced expiratory volume in one second (FEV 1 ) compared to patients treated with comparator anti-diabetes treatments. The decline occurred during the first 3 months of therapy and persisted over 2 years (Figure 2). A decline in FEV 1 of ≥ 15% occurred in 6% of AFREZZA-treated patients compared to 3% of comparator-treated patients [see Warnings and Precautions (5.4)].
Figure 2. Mean (+/-SE) Change in FEV 1 (Liters) from Baseline for Type 1 and Type 2 Diabetes Patients
Weight gain has occurred with some insulin therapies, including AFREZZA. Weight gain has been attributed to the anabolic effects of insulin and the decrease in glycosuria. In a clinical trial of patients with type 2 diabetes [see Clinical Studies (14.3)] , there was a mean 0.49 kg weight gain among AFREZZA-treated patients compared with a mean 1.13 kg weight loss among placebo-treated patients.
The following adverse reaction has been identified during post approval use of AFREZZA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: bronchospasm.
The risk of hypoglycemia associated with AFREZZA use may be increased with antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics. Dose modification and increased frequency of glucose monitoring may be required when AFREZZA is given concomitantly with these drugs.
The glucose lowering effect of AFREZZA may be decreased when given concomitantly with atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline) and thyroid hormones. Dose adjustment and increased frequency of glucose monitoring may be required when AFREZZA is given concomitantly with these drugs.
The glucose lowering effect of AFREZZA may be increased or decreased when co-administered with alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. Dose modification and increased frequency of glucose monitoring may be required when AFREZZA is given concomitantly with these drugs.
The signs and symptoms of hypoglycemia may be blunted when beta-blockers, clonidine, guanethidine, and reserpine are given concomitantly with AFREZZA.
Risk Summary Limited available data with AFREZZA use in pregnant women are insufficient to determine drug-associated risks for adverse developmental outcomes. Available information from published studies with human insulin use during pregnancy has not reported a clear association with human insulin and adverse developmental outcomes ( see Data ). There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy ( see Clinical Considerations ). In animal reproduction studies, there were no adverse developmental outcomes with subcutaneous administration of carrier particles (vehicle without insulin) to pregnant rats during organogenesis at doses 21 times the human daily dose of 99 mg AFREZZA, based on AUC (see Data) . The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with HbA1c >7 and has been reported to be as high as 20-25% in women with HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical ConsiderationsDisease-associated maternal and/or embryo/fetal risk Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, stillbirth, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, still birth, and macrosomia- related morbidity.
Human Data There are limited data with AFREZZA use in pregnant women. Published data do not report a clear association with human insulin and major birth defects, miscarriage, or adverse maternal or fetal outcomes when human insulin is used during pregnancy. However, these studies cannot definitely establish the absence of any risk because of methodological limitations including small sample size and lack of blinding.
Animal Data In pregnant rats given subcutaneous doses of 10, 30, and 100 mg/kg/day of carrier particles (vehicle without insulin) from gestation day 6 through 17 (organogenesis), no major malformations were observed at doses up to 100 mg/kg/day (21 times the human systemic exposure at a daily dose of 99 mg AFREZZA, based on AUC). In pregnant rabbits given subcutaneous doses of 2, 10, and 100 mg/kg/day of carrier particles (vehicle without insulin) from gestation day 7 through 19 (organogenesis), adverse maternal effects were observed in all dose groups (at human systemic exposure following a daily dose of 99 mg AFREZZA, based on AUC). In pregnant rats given subcutaneous doses of 10, 30, and 100 mg/kg/day of carrier particles (vehicle without insulin) from gestation day 7 through lactation day 20 (weaning), decreased epididymis and testes weights were observed in F1 male offspring, however, no decrease in fertility was noted, and impaired learning were observed in F1 pups at ³ 30 mg/kg/day (6 times the human systemic exposure at a daily dose of 99 mg AFREZZA, based on AUC).
Risk Summary There are no data on the presence of AFREZZA in human milk, the effects on the breastfed infant, or the effects on milk production. One small published study reported that exogenous subcutaneous insulin was present in human milk. No adverse effects in infants were noted. The carrier particles are present in rat milk ( see Data ). Potential adverse reactions that are related to inhalational administration of AFREZZA are unlikely to be associated with potential exposure of AFREZZA through breast milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for AFREZZA and any potential adverse effects on the breastfed infant from AFREZZA or from the underlying maternal condition.
Data Subcutaneous administration of the carrier particle in lactating rats resulted in excretion of the carrier particle in rat milk at levels that were approximately 10% of the maternal exposure. Given the results of the rat study, it is highly likely that the insulin and carrier in AFREZZA are excreted in human milk.
The safety and effectiveness of AFREZZA to improve glycemic control in pediatric patients with diabetes mellitus has not been established. AFREZZA has not been studied in pediatric patients.
In the AFREZZA clinical studies , 671 (12%) patients were 65 years of age or older, of which 42 (0.8%) were 75 years of age or older. In these studies, 381 (13%) of AFREZZA-treated patients were 65 years of age or older, of which 20 (0.7%) were 75 years of age or older. No overall differences in effectiveness of AFREZZA have been observed between patients 65 years of age and older and younger adult patients [see Clinical Studies (14)] . Clinical studies of AFREZZA did not include sufficient numbers of patients 65 years of age and older to determine whether there were differences in safety between these patients and younger adult patients. Pharmacokinetic and pharmacodynamic studies to assess the effect of age on pharmacokinetics or pharmacodynamics on insulin human, respectively, have not been conducted.
The effect of hepatic impairment on the pharmacokinetics of AFREZZA has not been studied. Frequent glucose monitoring and a lower dosage may be necessary in AFREZZA-treated patients with hepatic impairment [see Warnings and Precautions (5.3)] .
The effect of renal impairment on the pharmacokinetics of AFREZZA has not been studied. Some studies with human insulin have shown increased circulating levels of insulin in patients with renal failure. Frequent glucose monitoring and a lower dosage may be necessary in AFREZZA-treated patients with renal impairment [see Warnings and Precautions (5.3)] .
Excess insulin administration may cause hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.8)] . Mild episodes of hypoglycemia due to insulin overdose can usually be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise, may be needed. Severe episodes of hypoglycemia (due to insulin overdose) with coma, seizure, or neurologic impairment may be treated with intramuscular or subcutaneous glucagon or concentrated intravenous glucose. After apparent clinical recovery from hypoglycemia, continued observation and additional carbohydrate intake may be necessary to avoid recurrence of hypoglycemia. Hypokalemia should be corrected appropriately.
Human insulin is a rapid acting human insulin produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli (K12). Chemically, human insulin has the empirical formula C 257 H 383 N 65 O 77 S 6 and a molecular weight of 5808. Human insulin has the following primary amino acid sequence:
AFREZZA (human insulin) inhalation powder is available in single-use plastic cartridges filled with a white powder containing insulin (human), which is administered via oral inhalation using the AFREZZA Inhaler only. Insulin is adsorbed onto carrier particles consisting of fumaryl diketopiperazine (FDKP) and polysorbate 80. AFREZZA Inhalation Powder is a dry powder supplied as 4 unit, 8 unit or 12 unit cartridges.
The AFREZZA Inhaler is breath-powered by the patient. When the patient inhales through the device, the powder is aerosolized and delivered to the lung. The amount of AFREZZA delivered to the lung will depend on individual patient factors.
Insulin lowers blood glucose levels in adult patients with diabetes mellitus by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis in adipocytes, inhibits proteolysis, and enhances protein synthesis.
The time course of insulin action (i.e., glucose lowering) may vary considerably in different patients or within the same patient. The average pharmacodynamic profile [i.e., glucose lowering effect measured by glucose infusion rate (GIR) over time in a euglycemic clamp study] after administration of a single AFREZZA dose of 4, 12, and 48 units in 30 patients with type 1 diabetes is shown in Figure 3(A), and key characteristics regarding the timing of the effects are described in Table 4:
| Parameter for Insulin Effect | AFREZZA 4 units | AFREZZA 12 units | AFREZZA 48 units |
| Time to first measurable effect | ~12 minutes | ~12 minutes | ~12 minutes |
| Time to peak effect | ~35 minutes | ~45 minutes | ~55 minutes |
| Time for effect to return to baseline | ~90 minutes | ~180 minutes | ~270 minutes |
Figure 3. Results After Administration of AFREZZA 4, 12, and 48 Units in Patients with T1DM (N=30) A) Mean Insulin Effect (Baseline-Corrected Glucose Infusion Rate); and B) Pharmacokinetic (Baseline-Corrected Serum Insulin Concentration Profiles)
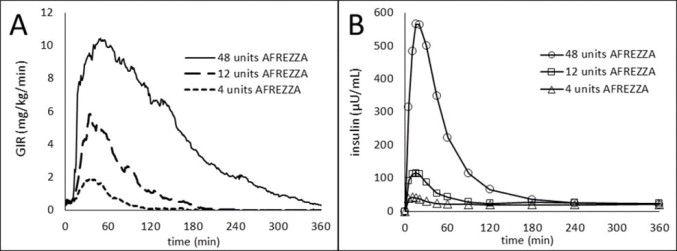
On average, the pharmacodynamics effect of AFREZZA, measured as area under the glucose infusion rate – time curve (AUC GIR) increased linearly with doses up to 48 units (106, 387, and 1581 mg/kg for 4, 12, and 48 units doses, respectively). Intrapatient variability in AUC GIR and GIR max was approximately 28% (95% CI 21-42%) and 27% (95% CI 20-40%), respectively.
The area under the plasma concentration versus time curve (AUC) of insulin increased dose proportionally up to 48 units. Intrapatient variability of AUC and peak concentration (C max ) of insulin was approximately 16% (95% CI 12-23%) and 21% (95% CI 16-30%), respectively.
Absorption The pharmacokinetic profiles for orally inhaled AFREZZA 4, 12, and 48 units from a study in 30 patients with type 1 diabetes are shown in Figure 5(B). The time to maximum serum insulin concentration (t max ) ranged from 10-20 minutes after oral inhalation of 4 to 48 units of AFREZZA.
Elimination The apparent terminal half-life ranged from 120 to 206 minutes. Serum insulin concentrations declined to baseline by approximately 60 to 240 minutes.
Metabolism and Excretion The metabolism and excretion of AFREZZA are comparable to regular human insulin.
Carrier Particles Clinical pharmacology studies showed that carrier particles [see Description (11.1)] are not metabolized and are eliminated unchanged in the urine following the lung absorption. Following oral inhalation of AFREZZA, a mean of 39% of the inhaled dose of carrier particles was distributed to the lungs and a mean of 7% of the dose was swallowed. The swallowed fraction was not absorbed from the GI tract and was eliminated unchanged in the feces.
Drug Interaction StudiesBronchodilators and Inhaled Steroids Albuterol increased the AUC insulin after AFREZZA administration by 25% in patients with asthma [see Drug Interactions (7.2)]. AFREZZA is contraindicated in patients with asthma. In a study in healthy volunteers no significant change in insulin exposure was observed when fluticasone was administered following AFREZZA administration.
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of insulin human or of other insulin human products. Increases in anti-insulin antibody concentrations were observed in patients treated with AFREZZA. Increases in anti-insulin antibodies were observed more frequently in patients treated with AFREZZA than in patients treated with subcutaneously injected mealtime insulin. There was no clinically significant effect of anti-drug antibodies on safety or effectiveness (as measured by HbA1c and fasting plasma glucose) of AFREZZA over the treatment duration of the studies which spanned 3 to 24 months.
In a 104 week carcinogenicity study, rats were given doses up to 46 mg/kg/day of the carrier and up to 1.23 mg/kg/day of insulin, by nose-only inhalation. No increased incidence of tumors was observed at systemic exposures equivalent to the insulin at a daily AFREZZA dose of 99 mg, based on a comparison of relative body surface areas across species. No increased incidence of tumors was observed in a 26 week carcinogenicity study in transgenic mice (Tg-ras-H2) given doses up to 75 mg/kg/day of carrier and up to 5 mg/kg/day of AFREZZA. AFREZZA was not genotoxic in Ames bacterial mutagenicity assay and in the chromosome aberration assay, using human peripheral lymphocytes with or without metabolic activation. The carrier alone was not genotoxic in the in vivo mouse micronucleus assay. In fertility study in male and female rats at subcutaneous doses of 10, 30, and 100 mg/kg/day of carrier (vehicle without insulin), there were no adverse effects on male fertility at doses up to 100 mg/kg/day. In female rats dosed 2 weeks prior to mating until gestation day 7, there was increased pre- and post-implantation loss at 100 mg/kg/day but not at 30 mg/kg/day (21 times and 6 times, respectively the human systemic exposure at a daily dose of 99 mg AFREZZA, based on AUC).
AFREZZA has been studied in adults with type 1 diabetes in combination with basal insulin. The efficacy of AFREZZA, in combination with basal insulin, in type 1 diabetes patients was compared to insulin aspart in combination with basal insulin. AFREZZA has been studied in adults with type 2 diabetes in combination with oral antidiabetic drugs. The efficacy of AFREZZA in type 2 diabetes patients was compared to placebo inhalation.
Patients with inadequately controlled type 1 diabetes participated in a 24-week, open-label, active-controlled study to evaluate the glucose lowering effect of mealtime AFREZZA used in combination with a basal insulin. Following a 4-week basal insulin optimization period, 344 patients were randomized to AFREZZA by oral inhalation (n=174) or insulin aspart given subcutaneously (n=170) at each meal of the day. All patients received basal insulin. Mealtime insulin doses were titrated to glycemic goals for the first 12 weeks and kept stable for the last 12 weeks of the study.
Results At Week 24, treatment with mealtime AFREZZA and basal insulin provided a mean reduction in HbA1c that met the pre-specified non-inferiority margin of 0.4%. AFREZZA and basal insulin provided less HbA1c reduction than insulin aspart and basal insulin, and the difference was statistically significant. More patients in the insulin aspart and basal insulin group achieved the HbA1c target of ≤7% (Table 5).
| a Adjusted mean was obtained using a Mixed Model Repeated Measures (MMRM) approach with HbA1c or FPG as the dependent variable and treatment, visit, region, basal insulin stratum, and treatment by visit interaction as fixed factors, and corresponding baseline as a covariate. An autoregression (1) [AR(1)] covariance structure was used. | ||
| b Data at 24 weeks were available from 131 (75%) and 150 (88%) patients randomized to the AFREZZA and insulin aspart groups, respectively. | ||
| c The percentage was calculated based on the number of patients randomized to the trial. | ||
| Efficacy Parameter | AFREZZA + Basal Insulin (N=174) | Insulin Aspart + Basal Insulin (N=170) |
| HbA1c (%) | ||
| Baseline (adjusted mean a ) | 7.94 | 7.92 |
| Change from baseline (adjusted mean a,b ) | -0.21 | -0.40 |
| Difference from insulin aspart (adjusted mean a,b ) (95% CI) | 0.19 (0.02, 0.36) | |
| Percentage of patients achieving HbA1c ≤ 7% c | 14% | 27% |
| Fasting Plasma Glucose (mg/dL) | ||
| Baseline (adjusted mean a ) | 153.9 | 151.6 |
| Change from baseline (adjusted mean a, b ) | -25.3 | 10.2 |
| Difference from insulin aspart (adjusted mean a, b ) (95% CI) | -35.4 (-56.3, -14.6) | |
A total of 479 adult patients with type 2 diabetes inadequately controlled on optimal/maximally tolerated doses of metformin only, or 2 or more oral antidiabetic (OAD) agents participated in a 24-week, double-blind, placebo-controlled study. Following a 6-week run-in period, 353 patients were randomized to AFREZZA by oral inhalation (n=177) or an inhaled placebo powder without insulin (n=176). Insulin doses were titrated for the first 12 weeks and kept stable for the last 12 weeks of the study. OADs doses were kept stable in the study.
Results At Week 24, treatment with AFREZZA plus OADs provided a mean reduction in HbA1c that was statistically significantly greater compared to the HbA1c reduction observed in the placebo plus OADs group (Table 6).
| a Adjusted mean was obtained using a Mixed Model Repeated Measures (MMRM) approach with HbA1c or FPG as the dependent variable and treatment, visit, region, and treatment by visit interaction as fixed factors, and corresponding baseline as a covariate. An autoregression (1) [AR(1)] covariance structure was used. | ||
| b Data at 24 weeks without rescue therapy were available from 139 (79%) and 129 (73%) patients randomized to the AFREZZA and placebo groups, respectively. | ||
| c The percentage was calculated based on the number of patients randomized to the trial. | ||
| Efficacy Parameter | AFREZZA + Oral Anti-Diabetic Agents (N=177) | Placebo + Oral Anti-Diabetic Agents (N=176) |
| HbA1c (%) | ||
| Baseline (adjusted mean a ) | 8.25 | 8.27 |
| Change from baseline (adjusted mean a,b ) | -0.82 | -0.42 |
| Difference from placebo (adjusted mean a,b ) (95% CI) | -0.40 (-0.57, -0.23) | |
| Percentage (%) of patients achieving HbA1C ≤7% c | 32% | 15% |
| Fasting Plasma Glucose (mg/dL) | ||
| Baseline (adjusted mean a ) | 175.9 | 175.2 |
| Change from baseline (adjusted mean a,b ) | -11.2 | -3.8 |
| Difference from placebo (adjusted mean a,b ) (95% CI) | -7.4 (-18.0, 3.2) | |
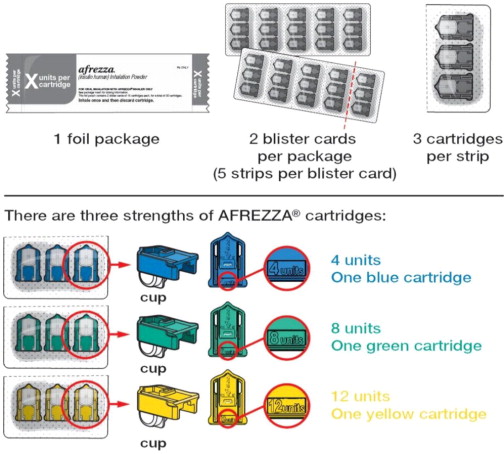
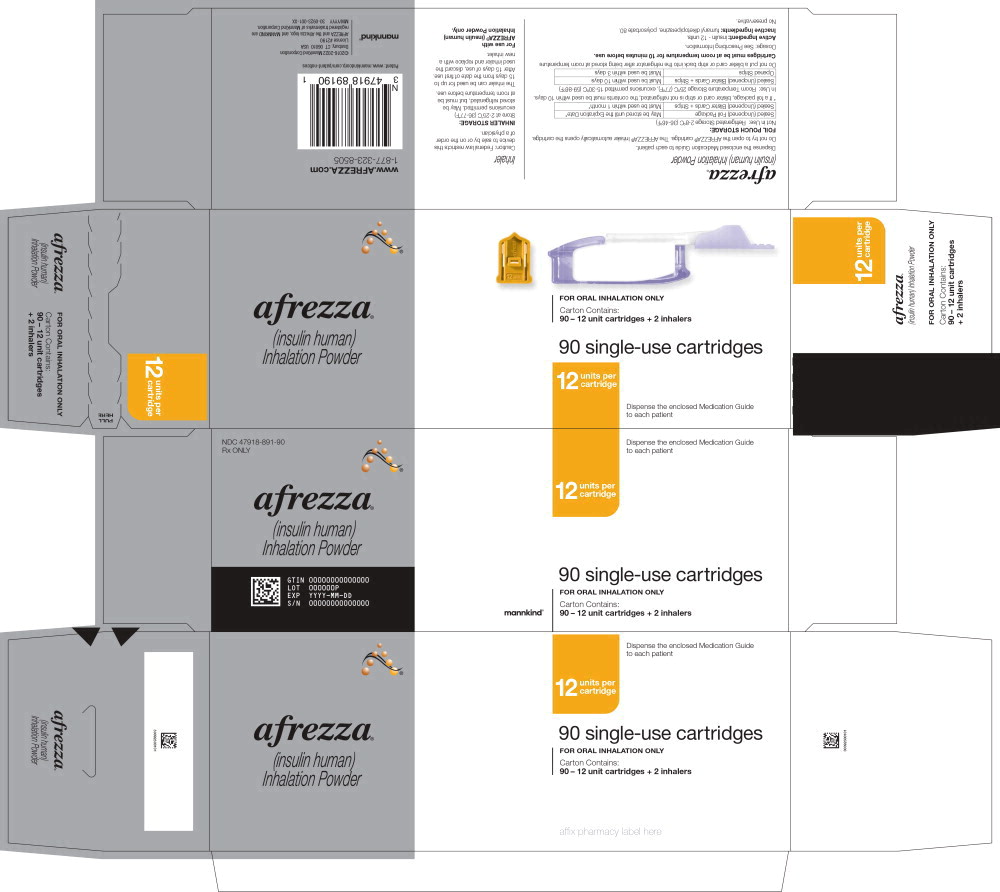
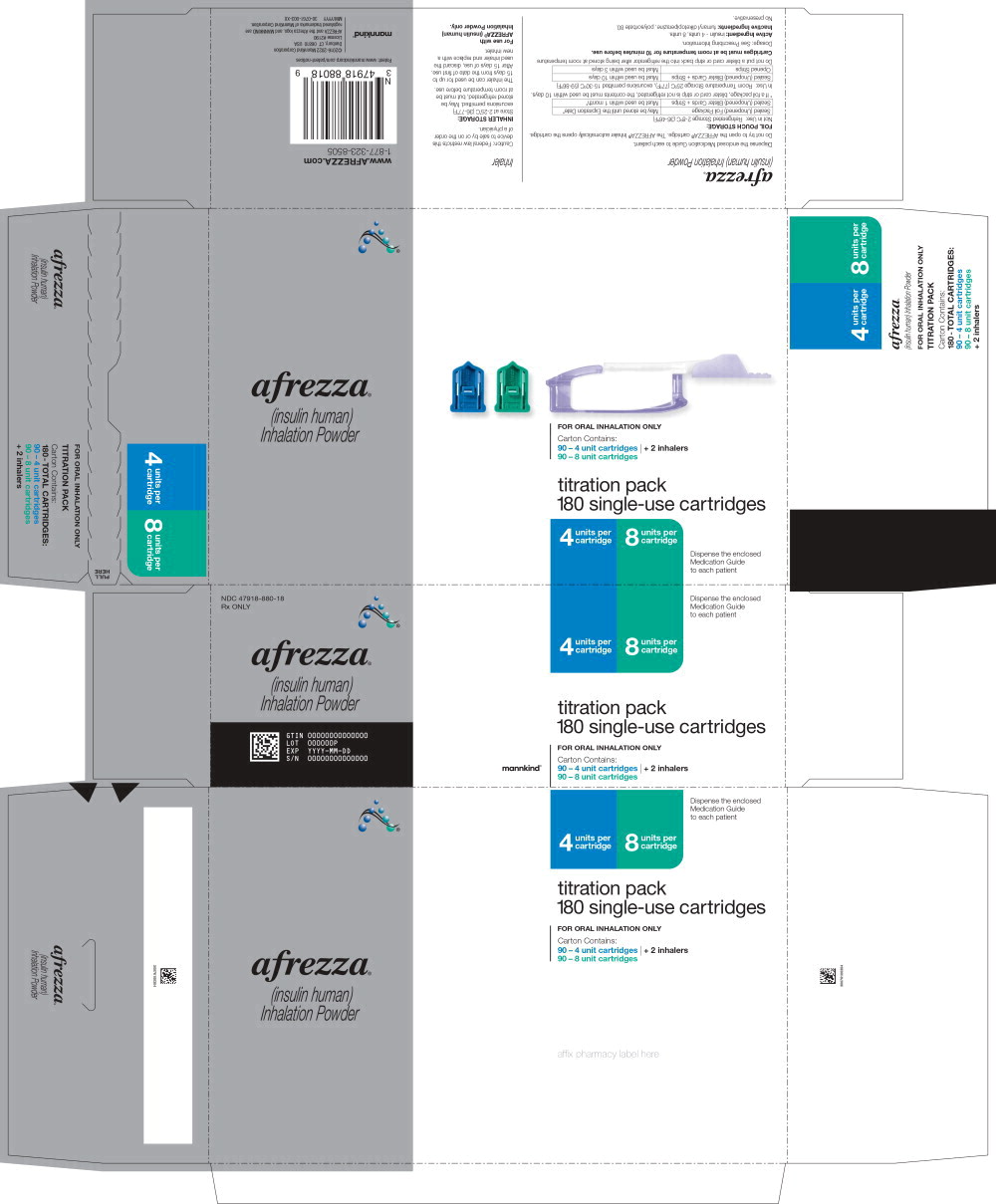
AFREZZA (insulin human) Inhalation Powder is available as 4 unit, 8 unit and 12 unit single-use cartridges. Three cartridges are contained in a single cavity of a blister strip. Each card contains 5 blister strips (each containing three cartridges) separated by perforations for a total of 15 cartridges. Two cards of the same cartridge strength are packaged in a foil laminate overwrap (30 cartridges per foil package). The cartridges are color-coded, blue for 4 units, green for 8 units and yellow for 12 units. Each cartridge is marked with “afrezza” and “4 units”, “8 units” or “12 units”. The AFREZZA Inhaler is individually packaged in a clear overwrap. The inhaler is fully assembled with a removable mouthpiece cover. The AFREZZA Inhaler can be used for up to 15 days from the date of first use. After 15 days of use, the inhaler must be discarded and replaced with a new inhaler. AFREZZA (insulin human) Inhalation Powder is available in the following configurations:
| NDC | Cartridge Strength | Quantity of Cartridges per Strength | Total Quantity of Cartridges per Kit | Total Units in Kit | Number of Inhalers |
| 47918-874-90 | 4 units | 90 | 90 | 360 Units | 2 |
| 47918-878-90 | 8 units | 90 | 90 | 720 Units | 2 |
| 47918-891-90 | 12 units | 90 | 90 | 1080 Units | 2 |
| 47918-898-18 | 8 units, 12 units | 90 | 180 | 1800 Units | 2 |
| 47918-880-18 (Titration Pack) | 4 units, 8 units | 90 | 180 | 1080 Units | 2 |
| 47918-902-18 (Titration Pack) | 4 units, 8 units, 12 units | 60 | 180 | 1440 Units | 2 |
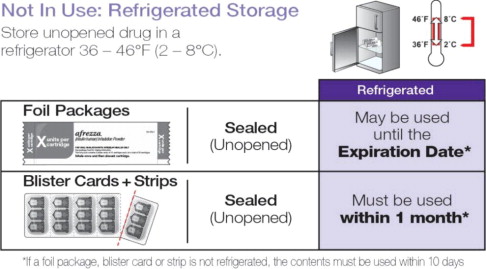
| * If a foil package, blister card or strip is not refrigerated, the contents must be used within 10 days. | |
| Sealed (Unopened) Foil Package | May be stored until the Expiration Date* |
| Sealed (Unopened) Blister Cards + Strips | Must be used within 1 month* |
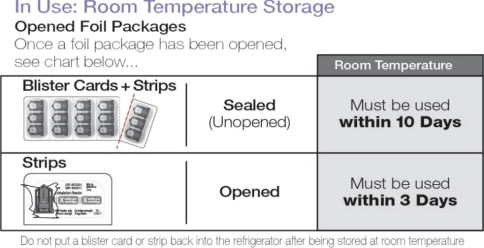
| Sealed (Unopened) Blister Cards + Strips | Must be used within 10 days |
| Opened Strips | Must be used within 3 days |
Do not put a blister card or strip back into the refrigerator after being stored at room temperature.
Inhaler Storage : Store refrigerated or at room temperature 2-25ºC (36-77ºF); excursions permitted. Inhaler may be stored refrigerated, but should be at room temperature before use.
Handling : Before use, cartridges should be at room temperature for 10 minutes.Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Instruct patients to use AFREZZA only with the AFREZZA inhaler.
Common Adverse Reactions Inform patients that the most common adverse reactions associated with the use of AFREZZA are hypoglycemia, cough, and throat pain or irritation.
Pregnancy Advise women with diabetes to inform their physician if they are pregnant or are planning to become pregnant while using AFREZZA.
Acute Bronchospasm in Patients with Chronic Lung Disease Advise patients that if they experience any respiratory difficulty after inhalation of AFREZZA, they should report it to their healthcare provider immediately for assessment.
Hypoglycemia Instruct patients on self-management procedures including glucose monitoring, proper inhalation technique, and management of hypoglycemia and hyperglycemia especially at initiation of AFREZZA therapy. Instruct patients on handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake, or skipped meals. Instruct patients on the management of hypoglycemia. Inform patients that their ability to concentrate and react may be impaired as a result of hypoglycemia. Advise patients who have frequent hypoglycemia or reduced or absent warning signs of hypoglycemia to use caution when driving or operating machinery [see Warnings and Precautions (5.3)].
Decline in Pulmonary Function Inform patients that AFREZZA can cause a decline in lung function [see Warnings and Precautions (5.4)].
Lung Cancer Inform patients to promptly report any signs or symptoms potentially related to lung cancer [see Warnings and Precautions (5.5)].
Diabetic Ketoacidosis Instruct patients to carefully monitor their blood glucose during illness, infection, and other risk situations for diabetic ketoacidosis and to contact their healthcare provider if their blood glucose control worsens [see Warnings and Precautions (5.6)].
Hypersensitivity Reactions Advise patients that hypersensitivity reactions can occur with insulin therapy including AFREZZA. Inform patients on the symptoms of hypersensitivity reactions [see Warnings and Precautions (5.7)]. Manufactured by: MannKind Corporation, Danbury, CT 06810
US License No. #2190
© 2016 – 2023 MannKind Corporation AFREZZA is a registered trademark of MannKind Corporation
Patent: www.mannkindcorp.com/patent-notices
Treatment with TZDs and AFREZZA may need to be changed or stopped by your healthcare provider if you have new or worse heart failure.
 |
| Figure A |
If you are having problems with your AFREZZA Inhaler or if it breaks and you need a new one, call 1-877-323-8505.
Know your AFREZZA Inhaler:
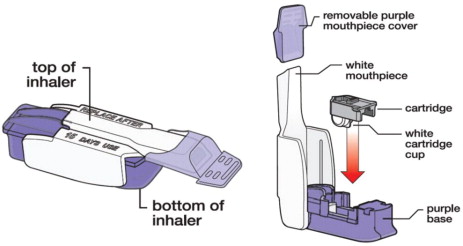
Know your AFREZZA cartridges:
| How to take your dose of AFREZZA: Always be sure you have the right number of AFREZZA cartridges for your dose available before you start. AFREZZA cartridges must only be used with the AFREZZA Inhaler. | |
| Step 1: Select the AFREZZA cartridges for your dose | |
 | |
| If your prescribed AFREZZA dose is more than 12 units you will need to use more than 1 cartridge to get your right dose. |
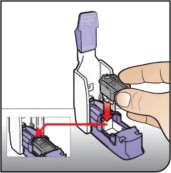
Hold the cartridge with the cup facing down.
Line up the cartridge with the opening in the inhaler. The pointed end of the cartridge should line up with the pointed end in the inhaler.
Place the cartridge into the inhaler. Be sure that the cartridge lies flat in the inhaler.
If any of these occur, throw away the cartridge
and load a new cartridge.
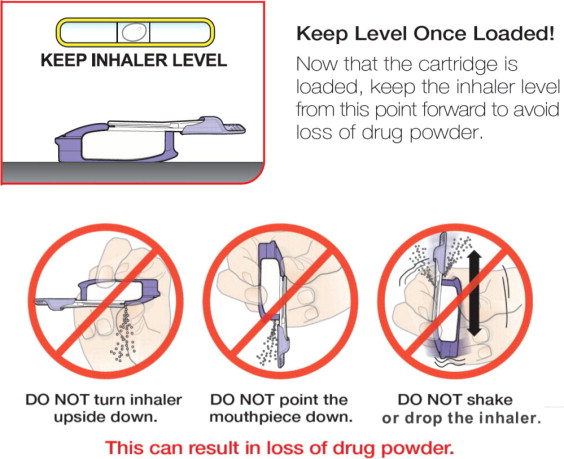
Keeping your head level, place the mouthpiece in your mouth and tilt the inhaler down towards your chin, as shown.
Close your lips around the mouthpiece to form a seal.
| Step 4: Removing a used cartridge | |
 | Replace Mouthpiece Cover |
| Multiple cartridge dosing | |
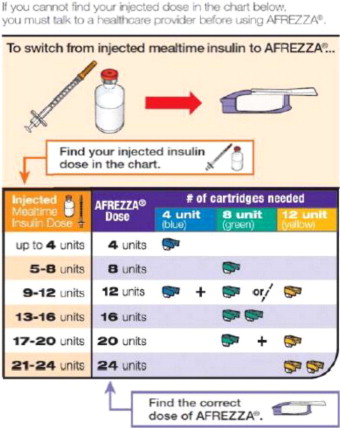
| If you need more than one (1) AFREZZA cartridge for your dose, see the AFREZZA dosage chart above (Figure B). | |
| How should I store AFREZZA? |
 |
 |
 |




Manufactured by: MannKind Corporation
Danbury, CT 06810
US License No. #2190
© 2016 – 2023 MannKind Corporation
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AFREZZA is a registered trademark of MannKind Corporation
Principal Display Panel 90 − 4 unit cartridges and 2 inhalers NDC 47918-874-90 Rx ONLY afrezza® (insulin human)
Inhalation Powder 4 units per
cartridge 90 single-use cartridges FOR ORAL INHALATION ONLY Carton Contains: 90 – 4 unit cartridges + 2 inhalers Dispense the enclosed Medication Guide
to each patient mannkind®

Principal Display Panel 90 – 8 unit cartridges and 2 inhalers NDC 47918-878-90 Rx ONLY afrezza® (insulin human)
Inhalation Powder 8 units per
cartridge 90 single-use cartridges FOR ORAL INHALATION ONLY Carton Contains: 90 – 8 unit cartridges + 2 inhalers Dispense the enclosed Medication Guide
to each patient mannkind®

Principal Display Panel 90 – 12 unit cartridges and 2 inhalers NDC 47918-891-90 Rx ONLY afrezza® (insulin human)
Inhalation Powder 12 units per
cartridge 90 single-use cartridges FOR ORAL INHALATION ONLY Carton Contains: 90 – 12 unit cartridges + 2 inhalers Dispense the enclosed Medication Guide
to each patient mannkind®
Principal Display Panel 180 cartridges; 60 – 4 unit cartridges, 60 – 8 unit cartridges and 60 – 12 unit cartridges and 2 inhalers (Titration Pack) NDC 47918-902-18 Rx ONLY afrezza® (insulin human)
Inhalation Powder 4 units per
cartridge 8 units per
cartridge 12 units per
cartridge 180 single-use cartridges FOR ORAL INHALATION ONLY Carton Contains: 60 – 4 unit cartridges 60 – 8 unit cartridges 60 – 12 unit cartridges 2 - inhalers Dispense the enclosed
Medication Guide
to each patient mannkind®

Principal Display Panel 180 cartridges; 90 – 4 unit cartridges and 90 – 8 unit cartridges and 2 inhalers (Titration Pack) NDC 47918-880-18 Rx ONLY afrezza® (insulin human)
Inhalation Powder 4 units per
cartridge 8 units per
cartridge titration pack 180 single-use cartridges FOR ORAL INHALATION ONLY Carton Contains: 90 – 4 unit cartridges 90 – 8 unit cartridges 2 - inhalers Dispense the enclosed
Medication Guide
to each patient mannkind®
Principal Display Panel 180 cartridges; 90 – 8 unit cartridges and 90 – 12 unit cartridges and 2 inhalers NDC 47918-898-18 Rx ONLY afrezza® (insulin human)
Inhalation Powder 8 units per
cartridge 12 units per
cartridge 180 single-use cartridges FOR ORAL INHALATION ONLY Carton Contains: 90 – 8 unit cartridges 90 – 12 unit cartridges 2 - inhalers Dispense the enclosed
Medication Guide
to each patient mannkind®

NDC 47918-903-27 Rx ONLY afrezza ® (insulin human)
Inhalation Powder 4 units per
cartridge 8 units per
cartridge 12 units per
cartridge 27 single-use cartridges FOR ORAL INHALATION ONLY Carton Contains: 9 – 4 unit cartridges 9 – 8 unit cartridges 9 – 12 unit cartridges 1 - inhaler Dispense the enclosed
Medication Guide
to each patient mannkind ®

| Product Information | |||
| Product Type | HUMAN PRESCRIPTION DRUG | Item Code (Source) | NDC:47918-874 |
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 4 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | NDC:47918-874-90 | 3 in 1 CARTON | 01/21/2015 | |
| 1 | 2 in 1 POUCH | |||
| 1 | 15 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 01/21/2015 | |
| Product Information | |||
| Product Type | HUMAN PRESCRIPTION DRUG | Item Code (Source) | NDC:47918-878 |
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 8 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | NDC:47918-878-90 | 3 in 1 CARTON | 07/01/2017 | |
| 1 | 2 in 1 POUCH | |||
| 1 | 15 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/01/2017 | |
| Product Information | |||
| Product Type | HUMAN PRESCRIPTION DRUG | Item Code (Source) | NDC:47918-891 |
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 12 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | NDC:47918-891-90 | 3 in 1 CARTON | 07/01/2017 | |
| 1 | 2 in 1 POUCH | |||
| 1 | 15 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/01/2017 | |
| Product Information | |||
| Product Type | HUMAN PRESCRIPTION DRUG | Item Code (Source) | NDC:47918-880 |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | NDC:47918-880-18 | 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package | 07/01/2016 | |
| Quantity of Parts | ||||
| Part # | Package Quantity | Total Product Quantity | ||
|---|---|---|---|---|
| Part 1 | 90 CARTRIDGE | 90 | ||
| Part 2 | 90 CARTRIDGE | 90 | ||
| Part 1 of 2 |
| AFREZZA insulin human powder, metered |
| Product Information | |||
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 8 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | 3 in 1 PACKAGE, COMBINATION | |||
| 1 | 2 in 1 POUCH | |||
| 1 | 15 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/11/2014 | |
| Part 2 of 2 |
| AFREZZA insulin human powder, metered |
| Product Information | |||
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 4 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | 3 in 1 PACKAGE, COMBINATION | |||
| 1 | 2 in 1 POUCH | |||
| 1 | 15 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/11/2014 | |
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/01/2016 | |
| Product Information | |||
| Product Type | HUMAN PRESCRIPTION DRUG | Item Code (Source) | NDC:47918-898 |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | NDC:47918-898-18 | 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package | 12/04/2018 | |
| Quantity of Parts | ||||
| Part # | Package Quantity | Total Product Quantity | ||
|---|---|---|---|---|
| Part 1 | 90 CARTRIDGE | 90 | ||
| Part 2 | 90 CARTRIDGE | 90 | ||
| Part 1 of 2 |
| AFREZZA insulin human powder, metered |
| Product Information | |||
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 8 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | 3 in 1 PACKAGE, COMBINATION | |||
| 1 | 2 in 1 POUCH | |||
| 1 | 15 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/11/2014 | |
| Part 2 of 2 |
| AFREZZA insulin human powder, metered |
| Product Information | |||
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 12 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | 3 in 1 PACKAGE, COMBINATION | |||
| 1 | 2 in 1 POUCH | |||
| 1 | 15 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/11/2014 | |
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 12/04/2018 | |
| Product Information | |||
| Product Type | HUMAN PRESCRIPTION DRUG | Item Code (Source) | NDC:47918-902 |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | NDC:47918-902-18 | 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package | 01/10/2017 | |
| Quantity of Parts | ||||
| Part # | Package Quantity | Total Product Quantity | ||
|---|---|---|---|---|
| Part 1 | 60 CARTRIDGE | 60 | ||
| Part 2 | 60 CARTRIDGE | 60 | ||
| Part 3 | 60 CARTRIDGE | 60 | ||
| Part 1 of 3 |
| AFREZZA insulin human powder, metered |
| Product Information | |||
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 8 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | 2 in 1 PACKAGE, COMBINATION | |||
| 1 | 2 in 1 POUCH | |||
| 1 | 15 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/11/2014 | |
| Part 2 of 3 |
| AFREZZA insulin human powder, metered |
| Product Information | |||
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 4 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | 2 in 1 PACKAGE, COMBINATION | |||
| 1 | 2 in 1 POUCH | |||
| 1 | 15 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/11/2014 | |
| Part 3 of 3 |
| AFREZZA insulin human powder, metered |
| Product Information | |||
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 12 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | 2 in 1 PACKAGE, COMBINATION | |||
| 1 | 2 in 1 POUCH | |||
| 1 | 15 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/11/2014 | |
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 01/10/2017 | |
| Product Information | |||
| Product Type | HUMAN PRESCRIPTION DRUG | Item Code (Source) | NDC:47918-903 |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | NDC:47918-903-27 | 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package | 11/09/2020 | |
| Quantity of Parts | ||||
| Part # | Package Quantity | Total Product Quantity | ||
|---|---|---|---|---|
| Part 1 | 9 CARTRIDGE | 9 | ||
| Part 2 | 9 CARTRIDGE | 9 | ||
| Part 3 | 9 CARTRIDGE | 9 | ||
| Part 1 of 3 |
| AFREZZA insulin human powder, metered |
| Product Information | |||
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 8 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | 9 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/11/2014 | |
| Part 2 of 3 |
| AFREZZA insulin human powder, metered |
| Product Information | |||
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 4 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | 9 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/11/2014 | |
| Part 3 of 3 |
| AFREZZA insulin human powder, metered |
| Product Information | |||
| Route of Administration | RESPIRATORY (INHALATION) | ||
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
|---|---|---|
| INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) | INSULIN HUMAN | 12 [arb'U] |
| Inactive Ingredients | |
| Ingredient Name | Strength |
|---|---|
| FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) | |
| POLYSORBATE 80 (UNII: 6OZP39ZG8H) | |
| Packaging | ||||
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
|---|---|---|---|---|
| 1 | 9 in 1 BLISTER PACK | |||
| 1 | 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package | |||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 07/11/2014 | |
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
|---|---|---|---|
| BLA | BLA022472 | 11/09/2020 | |
| Labeler - Mannkind Corporation (099981040) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Mannkind Corporation | 099981040 | MANUFACTURE(47918-874, 47918-878, 47918-891, 47918-880, 47918-898, 47918-902, 47918-903) , ANALYSIS(47918-874, 47918-878, 47918-891, 47918-880, 47918-898, 47918-902, 47918-903) , LABEL(47918-874, 47918-878, 47918-891, 47918-880, 47918-898, 47918-902, 47918-903) , PACK(47918-874, 47918-878, 47918-891, 47918-880, 47918-898, 47918-902, 47918-903) | |